Molecule Viewer
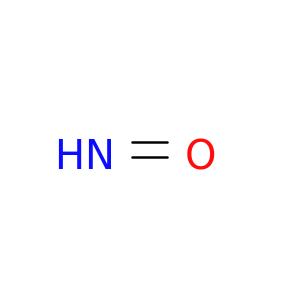
Thermodynamics at 298.15 K and standard pressure
| Enthalpy | ||
| Property | Value | Unit |
| Translational | 6.20 | kJ mol-1 |
| Rotational | 3.72 | kJ mol-1 |
| Vibrational | 41.85 | kJ mol-1 |
| Total (Trans. + Rot. + Vib.) | 51.77 | kJ mol-1 |
| Heat Capacity at Constant Pressure | ||
| Property | Value | Unit |
| Translational | 20.79 | J mol-1 K-1 |
| Rotational | 12.47 | J mol-1 K-1 |
| Vibrational | 0.35 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 33.61 | J mol-1 K-1 |
| Entropy | ||
| Property | Value | Unit |
| Translational | 151.58 | J mol-1 K-1 |
| Rotational | 67.60 | J mol-1 K-1 |
| Vibrational | 0.05 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 219.23 | J mol-1 K-1 |
| Other Properties | ||
| Property | Value | Unit |
| Heat of Formation | 57.77 | kJ mol-1 |
Vibrational Frequencies
Aqueous Solvation Energy at 298.15 K
| Surface |
|
| Dipole |
|
| Translucent Molecule |
|
| Property | Value | Unit |
| Total Solvation Energy | -7.46 | kJ mol-1 |
| Polar Solvation Energy | -12.37 | kJ mol-1 |
| Nonpolar Solvation Energy | 4.89 | kJ mol-1 |
| Surface Area | 51.96 | Å2 |
| Charge of Molecule | 0 | |
| Dipole | 1.98 | Debye |