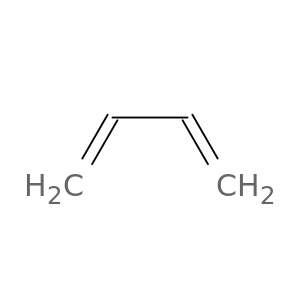
Buta-1,3-diene
Molecule Viewer
Thermodynamics at 298.15 K and standard pressure
| Enthalpy | ||
| Property | Value | Unit |
| Translational | 6.20 | kJ mol-1 |
| Rotational | 3.72 | kJ mol-1 |
| Vibrational | 224.63 | kJ mol-1 |
| Total (Trans. + Rot. + Vib.) | 234.55 | kJ mol-1 |
| Heat Capacity at Constant Pressure | ||
| Property | Value | Unit |
| Translational | 20.79 | J mol-1 K-1 |
| Rotational | 12.47 | J mol-1 K-1 |
| Vibrational | 42.52 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 75.78 | J mol-1 K-1 |
| Entropy | ||
| Property | Value | Unit |
| Translational | 158.51 | J mol-1 K-1 |
| Rotational | 98.59 | J mol-1 K-1 |
| Vibrational | 30.65 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 287.75 | J mol-1 K-1 |
| Other Properties | ||
| Property | Value | Unit |
| Heat of Formation | 129.71 | kJ mol-1 |
Molecular Orbitals
- 1 -300.18 eV View
- 2 -300.18 eV View
- 3 -299.76 eV View
- 4 -299.76 eV View
- 5 -28.17 eV View
- 6 -25.65 eV View
- 7 -20.98 eV View
- 8 -19.23 eV View
- 9 -16.18 eV View
- 10 -15.95 eV View
- 11 -13.53 eV View
- 12 -13.46 eV View
- 13 -11.90 eV View
- 14 -10.85 eV View
- 15 -7.21 eV View
- 16 6.73 eV View
- 17 11.41 eV View
- 18 15.77 eV View
- 19 17.96 eV View
- 20 18.61 eV View
- 21 18.88 eV View
- 22 19.27 eV View
- 23 23.60 eV View
- 24 25.21 eV View
- 25 27.48 eV View
- 26 29.60 eV View
Vibrational Frequencies
| Vibration |
|
| Vectors |
|
| Balls |
|
- 1 85.62 cm-1 View
- 2 334.84 cm-1 View
- 3 510.04 cm-1 View
- 4 530.47 cm-1 View
- 5 707.47 cm-1 View
- 6 914.97 cm-1 View
- 7 924.53 cm-1 View
- 8 942.45 cm-1 View
- 9 963.55 cm-1 View
- 10 1017.62 cm-1 View
- 11 1041.29 cm-1 View
- 12 1193.92 cm-1 View
- 13 1207.99 cm-1 View
- 14 1267.60 cm-1 View
- 15 1321.28 cm-1 View
- 16 1377.02 cm-1 View
- 17 1824.69 cm-1 View
- 18 1873.40 cm-1 View
- 19 3033.32 cm-1 View
- 20 3040.92 cm-1 View
- 21 3136.19 cm-1 View
- 22 3136.76 cm-1 View
- 23 3147.66 cm-1 View
- 24 3148.24 cm-1 View
Aqueous Solvation Energy at 298.15 K
| Surface |
|
| Dipole |
|
| Translucent Molecule |
|
| Property | Value | Unit |
| Total Solvation Energy | 12.03 | kJ mol-1 |
| Polar Solvation Energy | -2.67 | kJ mol-1 |
| Nonpolar Solvation Energy | 14.67 | kJ mol-1 |
| Surface Area | 77.58 | Å2 |
| Charge of Molecule | 0 | |
| Dipole | 0.00 | Debye |