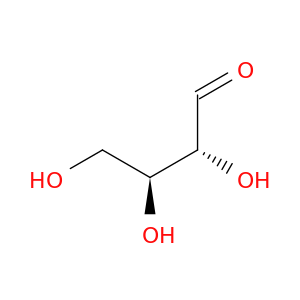
(2r,3s)-2,3,4-trihydroxybutanal
Molecule Viewer
Thermodynamics at 298.15 K and standard pressure
| Enthalpy | ||
| Property | Value | Unit |
| Translational | 6.20 | kJ mol-1 |
| Rotational | 3.72 | kJ mol-1 |
| Vibrational | 348.66 | kJ mol-1 |
| Total (Trans. + Rot. + Vib.) | 358.58 | kJ mol-1 |
| Heat Capacity at Constant Pressure | ||
| Property | Value | Unit |
| Translational | 20.79 | J mol-1 K-1 |
| Rotational | 12.47 | J mol-1 K-1 |
| Vibrational | 107.80 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 141.06 | J mol-1 K-1 |
| Entropy | ||
| Property | Value | Unit |
| Translational | 168.46 | J mol-1 K-1 |
| Rotational | 118.81 | J mol-1 K-1 |
| Vibrational | 91.00 | J mol-1 K-1 |
| Total (Trans. + Rot. + Vib.) | 378.26 | J mol-1 K-1 |
| Other Properties | ||
| Property | Value | Unit |
| Heat of Formation | -729.82 | kJ mol-1 |
Vibrational Frequencies
| Vibration |
|
| Vectors |
|
| Balls |
|
- 1 66.09 cm-1 View
- 2 99.15 cm-1 View
- 3 164.50 cm-1 View
- 4 208.95 cm-1 View
- 5 302.55 cm-1 View
- 6 355.93 cm-1 View
- 7 383.76 cm-1 View
- 8 390.16 cm-1 View
- 9 401.13 cm-1 View
- 10 412.15 cm-1 View
- 11 521.90 cm-1 View
- 12 574.54 cm-1 View
- 13 614.03 cm-1 View
- 14 647.46 cm-1 View
- 15 822.51 cm-1 View
- 16 919.58 cm-1 View
- 17 938.63 cm-1 View
- 18 1009.72 cm-1 View
- 19 1053.97 cm-1 View
- 20 1065.84 cm-1 View
- 21 1095.16 cm-1 View
- 22 1108.83 cm-1 View
- 23 1124.77 cm-1 View
- 24 1208.96 cm-1 View
- 25 1225.16 cm-1 View
- 26 1237.01 cm-1 View
- 27 1301.41 cm-1 View
- 28 1331.11 cm-1 View
- 29 1338.94 cm-1 View
- 30 1362.74 cm-1 View
- 31 1376.76 cm-1 View
- 32 1401.46 cm-1 View
- 33 1474.40 cm-1 View
- 34 1979.90 cm-1 View
- 35 2897.61 cm-1 View
- 36 2903.39 cm-1 View
- 37 2935.31 cm-1 View
- 38 2957.12 cm-1 View
- 39 3019.81 cm-1 View
- 40 3749.79 cm-1 View
- 41 3863.10 cm-1 View
- 42 3883.40 cm-1 View